18. Kontopoulos, D.-G.✉, Patmanidis, I., Barraclough, T., and Pawar, S. (2025). Changes in flexibility but not in compactness underlie the thermal adaptation of prokaryotic adenylate kinases. Evolution Letters 9(5):598-609. 
Abstract:
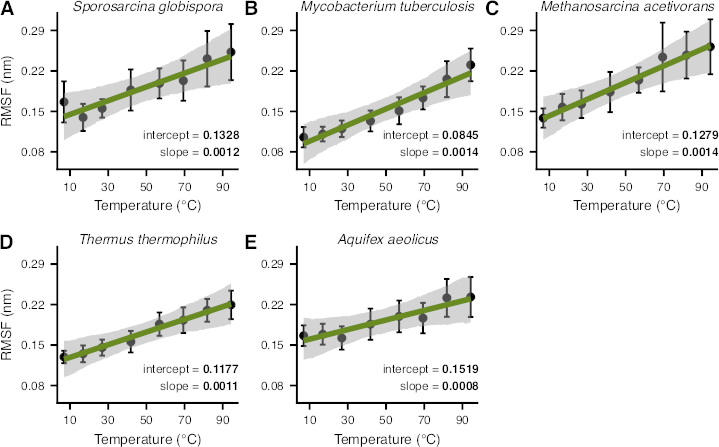
Understanding the structural changes that enable enzymes to remain active in extreme thermal conditions is of broad scientific interest for both fundamental and applied biological research. Three key mechanisms that underlie the thermal adaptation of enzymes are modifications in structural flexibility, compactness, and the contacts formed among amino acids. However, most previous studies on these topics have been limited to small sample sizes or a narrow taxonomic focus, and the importance of these factors to thermal adaptation remains poorly understood. In this study, we combined molecular dynamics simulations and phylogenetic comparative analyses to thoroughly analyze the structural factors underlying thermal adaptation in adenylate kinase—a key enzyme involved in cellular energy balance and homeostasis—across 70 prokaryotic species. We detect systematic increases in the flexibility of the enzyme with temperature, both across and within species. In contrast, structural compactness appears to be almost completely independent of temperature. Finally, we uncover a remarkable diversity in the number and types of amino acid contacts observed in different adenylate kinases that cannot be explained solely by temperature. Our results suggest that there are multiple paths toward the adaptation of prokaryotic adenylate kinases to extreme thermal environments and that these paths are generally accessible through changes in flexibility.
 citation
citation